EpiCARE Research Council – Current Grant Opportunities
Ongoing research calls
- Research (Fundamental, Clinical & Technologies)
- Travel & Mobility Grants, Doctoral programs
- Other (Prize, Networking, Infrastructure, workshop funding…)
Research (Fundamental, Clinical & Technologies)
Budget: The available budget for this call is 35 Mio. € (approx.).

Aims of the Call
The overall objectives of the JTC2025 will be to
- Support research projects in human health on pharmacogenomic strategies for personalised medicine approaches that address one or more of the following aspects:
- identification of new pharmacogenomic markers or signatures using (multi)-omics data in relation to drug or drug combination.
- validation of a pharmacogenomic marker or signatures using (multi)-omics data in predicting drug or drug combination outcomes.
- use pharmaco-omics strategies to determine the right dosage, the efficacy of treatments and/or the risk of adverse drug response and non-response to treatment to tailor personalised treatment pathways, including combined treatments (multi-medication).
- Encourage and enable interdisciplinary collaborations, i.e. multi-actor research by engaging a range of other relevant disciplines such as pre-clinical and clinical research, bioinformatics/health informatics/data research, ELSA research, implementation research or health economics research connected to the proposed research topic, including end-user perspective analysis to support the implementation of the research outcomes into clinical practice.
- Encourage cross-sectorial collaborations, by including the private sector (e.g. SMEs, small and medium-sized enterprises), industry, as well as regulatory/HTA agencies and patient organisations.
- Establish participatory research, i.e. active representation of patients or citizens as part of research projects.
Research projects in all disease areas will be welcome.
General (Eligibility) Conditions for Application
Whilst applications will be submitted jointly by groups from several countries, the individual applicants of each group will be funded by the respective regional/national EP PerMed funding organisation of the individual applicants. The applications are therefore subject to eligibility criteria and regulations of individual funding organisations.
Joint research proposals may be submitted by applicants belonging to the following categories (subject to regional/national funding regulations):
- Academia (research teams working in universities, other higher education institutions) or research institutes;
- Clinical/public health sector (research teams working in hospitals/public health and/or other healthcare settings and health organisations). Participation of clinicians (e.g. medical doctors, nurses) in the research teams is encouraged;
- Private for-profit (industry) partners, e.g. SME (small and medium-sized enterprises) and private non-profit partners, e.g. foundations, associations or non-governmental organisations.
Budget: Projects may be funded at the $25,000, $50,000, or $75,000 level. The project duration is one or two years.
No deadline
The Lennox-Gastaut Syndrome Foundation (LGS Foundation) is a non-profit organisation based in the United States. Its mission is to improve the lives of individuals affected by Lennox-Gastaut Syndrome, a rare form of childhood-onset epilepsy, through research, programmes and education. Projects may be on any novel topic in LGS but must be directly relevant to LGS, which is characterized by specific seizure types and hallmark EEG features.
The LGS Foundation Cure LGS 365 Research Grants provide funding to seed new basic, translational, and clinical Lennox-Gastaut Syndrome research projects.
Applications must be submitted by email. Letters of Intent (LOIs) may be submitted at any time; there are no deadlines. Selected applicants are invited to submit a full proposal.
Are you a scientist who wants to consolidate your independence by establishing a research team and continuing to develop a success career in Europe? The ERC Consolidator Grant could be for you. You can also apply if you have recently created an independent, excellent research team and want to strengthen it.
Budget: up to € 2 million for a period of 5 years. (pro rata for projects of shorter duration).
However, an additional € 1 million can be made available to cover eligible “start-up” costs for researchers moving from a third country to the EU or an associated country and/or the purchase of major equipment and/or access to large facilities and/or other major experimental and field work costs.
Deadline: 14 January 2025
Who can apply? Researchers of any nationality with 7-12 years of experience since completion of PhD, a scientific track record showing great promise and an excellent research proposal can apply.
Criteria: Applications can be made in any field of research.
Budget: 3000000 to 4000000 euros
Deadline: 26 November 2024
This topic aims at supporting activities that are enabling or contributing to one or several expected impacts of destination 3 “Tackling diseases and reducing disease burden”. To that end, proposals under this topic should aim for delivering results that are directed, tailored towards and contributing to some of the following expected outcomes:
- Health care practitioners and providers in low- and middle-income countries (LMICs) and/or those in high-income countries (HICs) serving disadvantaged populations have access to and use specific guidelines to implement health interventions that improve the availability of effective, equitable, efficient, integrated, patient-centred, safe, and timely care and the overall quality of life for people living with multiple long-term conditions including non-communicable diseases (NCDs).
- Public health managers and authorities, including from other relevant sector (e. g., social, culture) have access to improved insights and evidence on how to decrease the fragmentation of care for patients living with multiple chronic conditions, and ensure continuity of care across all stages of disease progression. They use this knowledge to design policies to reduce health inequities.
- Adopting an implementation science approach to studying interventions for management of multiple long-term conditions in the context of NCDs, researchers, clinicians and authorities have an improved understanding how the proposed interventions could be adopted in LMICs and/or disadvantaged populations of HICs setting, taking into account specific social, political, economic and cultural contexts.
- Communities and local stakeholders and authorities are fully engaged in implementing and taking up interventions for management of multiple long-term conditions in the context of NCDs and thus contribute to deliver better health.
Applicants must explore the implementation of proposed intervention(s) for a selected study population(s) taking into account the unique social, political, economic, and cultural context(s) in which the study will take place. Applicants should justify why any adaptation will not compromise the known effectiveness of the selected intervention(s).
Proposals should address all of the following activities[4]:
- Provide a research plan using validated implementation research frameworks or hybrid design research;
- Have an appropriate strategy for measuring implementation research outcomes and real-world effectiveness outcomes and indicators;
- Specifically address health equity and the principles of Universal Health Coverage[5];
- Engage an appropriately expert and skilled research team which can ensure a suitable multidisciplinary approach and that demonstrates equitable partnership and shared leadership between HIC-LMIC, and/or non-Indigenous–Indigenous members of the project team and external stakeholders through a clear governance strategy;
- Provide a stakeholder engagement strategy with evidence of support/engagement from key stakeholders for delivering patient-centred care and a pathway to sustain the proposed intervention after the funding ends;
- Provide opportunities for implementation research capacity building for early career researchers and team members from lower resourced environments, such as LMICs or disadvantaged communities.
- Ensure meaningful involvement of early career team members, including at least one early career member as a co-investigator.
Applicants are also encouraged to follow a life course approach, adapting the intervention to one or more key life stage(s) critical for reducing the onset or progression of MLTC NCD, and to explore how to best implement digital technology interventions.
The following are potential interventions or strategies that applicants may consider in their implementation plan (please note that this is not an exhaustive list):
- Strategies for improving MLTC NCD identification, stratification/staging, management, and/or monitoring such as investigating strategies for adapting and implementing the protocol(s) described in the WHO Package of Essential NCD Interventions (WHO PEN)[6] that address MLTC NCD management. For example, projects may focus on integrating NCD care into clinics that typically focus on the management of infectious diseases, such as HIV or tuberculosis clinics, or the integration of NCD care into maternal and child health clinics;
- Strategies to streamline and improve quality of care among individuals with MLTC NCD to reduce fragmentation of services, including task-sharing and/or the use of clinical decision-making tools (e.g., digital tools);
- Strategies and/or tools (e.g., digital tools) that optimise appropriate medication and (non-pharmacological) therapeutic prescribing, adherence, and/or reduced drug interactions/ adverse effects;
- Interventions that improve transitions through the health system, from community to primary to tertiary care and beyond, such as to home care or hospice;
- Health behavioural change interventions that target different risk factor clusters (e.g., exercise, nutrition, tobacco, alcohol and substance abuse).
Deadlines: Project applications can be submitted until
- 1 February for the summer meeting of the foundation committees in June
- 1 September for the winter meeting of the foundation committees in February
of each year.
In accordance with one of the desires of the donors, special attention is devoted by the foundation institutions to medical research. At present the foundation is focusing its support on the field of “Molecular causes in the development of illnesses”. This programme supports molecular biological studies of illnesses whose development is based on genetic defects or with which gene variants contribute to the development of complex illnesses.
In the area of “Molecular causes in the development of illnesses”, molecular biological studies of illnesses are supported whose development is primarily based on genetic defects or whose gene variants contribute to the development of complex illnesses.
Studies may be performed on cell culture and/or animal models, but should at least in part be performed on human tissue specimens and/or cells bearing relevance to illnesses. This only applies for applications submitted for support of projects.
- Project proposals are only accepted from researchers that have a Ph.D. and relevant experience in the priority field of research. They should generally have two to four years’ experience in post-doctoral research and want to set up or expand a small working group of their own with the support of the Foundation. The position of the applicant should be funded by the host research Institute.
- It is generally not possible to obtain funding for one’s own position.
- Firmly established researchers and scientists (holders of chairs, directors of clinics) are not eligible for support of projects.
The following projects are assigned preference:
- The functional analysis of genes, gene products and their signal transduction pathways for monogenic and complex genetic illnesses in vitro and in vivo, whereby the work plan should also contain studies on human tissue specimens and/or cells
- The characterisation of cell and animal models that have already been established for the study of genetic illnesses (with molecular biology methods)
- The analysis of predisposing genes or therapies which modify illnesses (‘personalised medicine’) if this holds out the promise of additional findings being generated on the mechanistic causes of disease formation
Support is not provided for:
- Purely methodological studies
- Descriptive genetic studies of populations as well as linkage and association studies
- Purely drug screening projects
- Research projects without any direct connection to an illness
- Research projects involving the development of an animal model that is to be studied in the grant period applied for
- Research projects that do not involve any studies on human tissue specimens and/or cells bearing relevance to illnesses
- Research projects relating to infectious diseases
Diagnostic and primarily therapy-oriented projects
TYPES OF COSTS
PERSONNEL COSTS
Complete personnel costs can be applied for in the case of research staff with doctoral degrees under
TVL-E13. In the case of research staff who do not have doctoral degrees, their pay is generally based on
65% of a TVL-E13-position. In countries other than Germany, the personnel costs are to be stated
according to the salary costs arrangements applicable there, i.e. total wage costs including the employer’s
share as a total sum.
In the case of applying for personnel costs for clinician scientists, the foundation assumes that the
planned staff will be put on leave at least 80 per cent of their working time at the clinic. Confirmation of
this from the clinic is to be submitted with the application.
Non-academic personnel are paid at the appropriate TVL level.
Student helpers or research assistants should be paid according to the rates prevailing at the institution
involved; the foundation is to be notified hereof through the budget plan accompanying the application.
TRAVEL COSTS
Funds to defray travel costs that are directly connected with the project can be applied for. In addition to
reasonable travel and overnight accommodation costs, up to € 28 a day can be granted to defray the costs
of meals when traveling within Germany (rail travel 2nd class or air travel economy class).
In the case of travel abroad, the daily or monthly rates for the respective country of travel can be applied
for to defray overnight accommodation and meal costs.
WHAT CANNOT BE APPLIED FOR
› Staff resources for the own post
› Financial resources for construction work
› Financial resources to procure office furnishings and equipment that are usually part of the basic
furnishings at universities and institutes
› Generally, no financial resources are granted to defray overhead costs
The Global Innovation Fund (GIF) is currently inviting applications for its Grants program to support breakthrough solutions from for-profit firms, non-profit organisations, researchers, and government agencies to maximise their impact and catalyse meaningful change.
Deadline – Ongoing
The Global Innovation Fund invests in the development, rigorous testing, and scaling up of new products, services, business process, or policy reforms. Through its grants, GIF supports these breakthrough solutions from for-profit firms, non-profit organisations, researchers, and government agencies to maximise their impact and catalyse meaningful change.
If you are a non-profit and your innovation does not involve generating revenues from users or customers, a grant is likely to be most appropriate.
Stages of Funding
GIF has a staged funding approach, whereby the amount of funding available is tiered according to the level of maturity of your innovation and the activities proposed. The three tiers are:
Pilot – the innovation is at an early stage but you have a credible plan for how it can be developed and tested in a real-world setting. Funding of up to USD 230,000 is available to test core assumptions around operational, social, and financial viability.
Test and transition – the innovation has already shown promise of success at a small scale, and you have some information on your operational, social, and financial viability which you want to solidify before you scale. Funding of up to USD 2.3 million is available to support further growth and generate additional evidence on whether the innovation can achieve social impact and market viability, for commercial innovations.
Scale – the innovation has a strong evidence base and logistically credible plan for scaling to reach millions of people. Funding of up to USD 15 million is available to expand the reach of innovations with a view to reaching millions of people in the long term if successful.
What they Fund?
At GIF, they believe that innovation, by which they mean any solution that has potential to address an important development problem more effectively than existing approaches, can come from anyone, anywhere.
This means that they accept applications working in any sector in any developing country.
Any type of organisation may apply. This includes social enterprises, for-profit companies, non-profit organisations, government agencies, international organisations, and research institutions in any country. It is recommended that individual innovators, entrepreneurs, or researchers apply through an affiliated organisation.
Eligibility Criteria
Any type of organisation may apply. This includes social enterprises, for-profit companies, non-profit organisations, government agencies, international organisations, and research institutions in any country. It is recommended that individual innovators, entrepreneurs or researchers apply through an affiliated organisation.
Ineligible
GIF is open to innovations which meet their criteria in any sector or country. However, there are some activities they do not fund. These include:
- Theoretical research, or purely lab-based activities that are not linked to implementation of a specific proposed real-world pilot or demonstration project.
- Approaches that are only applicable in a single country (unless the innovation is expected to scale to a large proportion of one of the world’s most populous developing countries).
The FamilieSCN2A Hodgkin-Huxley Grant program was created to honor the achievements of Dr. Alan Hodgkin and Dr. Andrew Huxley and their innovative modeling of action potentials, as well as their contributions which laid the groundwork for neuroscience research on the molecular, cellular, and circuit levels.
Unsolicited, year-round LOIs accepted. Full application invitations on a rolling basis as long as funds are available.
These research grant awards are intended for established, experienced, independent investigators affiliated with a research or academic institution whose proposed projects seek to investigate hypotheses directly related to hypothalamic hamartoma syndrome. Proposals are scored based on the quality of preliminary data, research design, feasibility, investigator’s qualifications, and overall impact.
Investigators applying for a research grant should ensure their proposed project addresses the needs of the hypothalamic hamartoma syndrome community and Hope for HH’s mission to support research toward better understanding, improved treatments and ultimately a cure for hypothalamic hamartoma syndrome.
Eligibility:
Applicants should be affiliated with a research or academic institution (excluding for-profit companies), may be US or foreign based, established in their field, and in good standing with their institution.
Our research priority areas include:
- Research that helps identify patient trends, characteristics, epidemiology, or other clinical aspects of hypothalamic hamartoma syndrome and/or its comorbidities.
- Research that will encourage the development of novel therapies to eliminate or prevent seizure progression or halt the progression of other comorbidities associated with hypothalamic hamartoma syndrome.
- Research that helps to understand, predict, and prevent SUDEP
Link: will be updated
Deadline: The call will be launched in early 2025; the deadline date will be provided once announced.
Indicative budget
Applicant consortia will be competing for the maximum financial contribution from IHI JU of up to EUR 20 000 000. NB: this amount is indicative and subject to change, pending approval by the IHI Governing Board.
IHI JU estimates that an IHI JU financial contribution of EUR 5 000 000 to 10 000 000 would allow a proposal to address these outcomes appropriately. NB: this amount is indicative and subject to change, pending approval by the IHI Governing Board. Nonetheless, this does not preclude the
submission and selection of a proposal requesting different amounts
Scope:
With a view to harnessing new science and technologies, this topic aims to fund pre-competitive research and innovation for novel tools, methods, technologies etc. that will foster the development of health innovations to prevent, intercept, diagnose, treat, and manage diseases and enable recovery more efficiently.
Accordingly, applicants must assemble a collaborative public-private partnership consortium reflecting the integrative and cross-sectoral nature of IHI JU, and capable of addressing the challenge(s) and scope of the IHI JU Specific Objective 1 “contribute towards a better understanding of the determinants of health and priority disease areas”
Applicants are encouraged to use the opportunity offered by emerging industrial technologies (e.g. innovative imaging methods, robotics or artificial intelligence) to provide better targets and approaches to develop new and more precise personalised health innovations for prevention, diagnosis and therapy, as well as facilitating good health while aging.
Applicants should consider the following points in their proposals:
- a) address an unmet public health need based on at least one of the below:
- the high burden of the disease for patients and/or society due to its severity and/or the number of people affected by it;
- the high economic impact of the disease for patients and society;
- the transformational nature of the potential results on innovation processes where projects are not focused on individual disease areas (e.g. health data analytics).
- b) demonstrate the ability to translate research into innovative solutions that can be integrated/implemented into the healthcare ecosystem and/or in industrial processes.
Expected impacts to be achieved by this topic
The actions to be funded under this topic are expected to achieve the following:
- a) contribute to one or more of IHI JU’s expected impacts linked to Specific Objective 1 as set out in the IHI JU SRIA, i.e.:
- patients benefit from preventive treatment or early disease intervention before onset of symptoms;
- prevention and early diagnosis of disease combined with better understanding of the mechanisms involved, leading to the development of more cost-effective strategies;
- patients benefitting from improved healthcare through regular monitoring of critical parameters using validated tools; development of new vaccine strategies targeted to specific sub-populations;
- increased preparedness of EU healthcare systems for disease outbreaks
- b) contribute to strengthening the competitiveness of the EU’s health industry, via increased economic activity in the development of health technologies, in particular, integrated health solutions, thus fostering European technological leadership and the digital transformation of our societies.
Link : will be updated
Deadline: The call will be launched in early 2025; the deadline date will be provided once announced.
Indicative budget
Applicant consortia will be competing for the maximum financial contribution from IHI JU of up to EUR 80 000 000. NB: this amount is indicative and subject to change, pending approval by the IHI Governing Board.
IHI JU estimates that an IHI JU financial contribution of EUR 8 000 000 to 15 000 000 would allow a proposal to address these outcomes appropriately. NB: this amount is indicative and subject to change, pending approval by the IHI Governing Board. Nonetheless, this does not preclude the submission and selection of a proposal requesting different amounts.
Scope:
With a view to harnessing new science and technologies, this topic aims to fund pre-competitive research and innovation for novel tools, methods, technologies etc. that will foster the development of health innovations to prevent, intercept, diagnose, treat, and manage diseases and enable recovery more efficiently.
Accordingly, applicants must assemble a collaborative public-private partnership consortium
Proposals could aim, for example, to break down fragmentation between various disciplines of medicine including computational and technological areas to accelerate innovations from early discovery to patient treatment.
Among others, proposals may aim to integrate diverse components (e.g. from focused mission-based research projects, collaborative platforms, databases, AI/ML to diagnostics, medicinal products, medical devices, wearables, digital solutions) in order to foster the development of people-centred, ambitious, large-scale and transformative solutions along the healthcare pathway from beginning to end, including treatment discovery.
Activities may include novel and harmonised approaches to data generation and federation, algorithm optimisation and applicable ML outputs, as well as activities to deliver open-source computational outputs such as machine learning methods for prediction at scale derived from a collaborative, community driven ecosystem.
Other examples are activities that catalyse data-driven AI/ML-influenced discoveries and therapies (e.g. integration of in vitro, in vivo approaches), (small molecules) screening platforms, manufacturing processes such as mass protein expression, diagnostics and prognostics for early and adapted treatment, including multimodal disease and/or cross-therapy area applications or management approaches. Proposals may address specific target populations, and/or support challenging unmet needs and treatment gaps.
Applicants should consider the following points in their proposals:
- a) address an unmet public health need based on at least one of the below:
- the high burden of the disease for patients and/or society due to its severity and/or the number of people affected by it;
- the high economic impact of the disease for patients and society;
- the transformational nature of the potential results on innovation processes where projects are not focused on individual disease areas (e.g. health data analytics).
- b) demonstrate the ability to translate research into innovative solutions that can be integrated/implemented into the healthcare ecosystem and/or in industrial processes.
Expected impacts to be achieved by this topic
The actions to be funded under this topic are expected to achieve the following:
- a) contribute to one or more of IHI JU’s expected impacts linked to the IHI JU’s Specific Objective 2, as set out in the IHI JU SRIA, i.e.
- breaking down fragmentation between various disciplines of medicine and technological areas in order to conceive and develop technologically and socially innovative, people- centred, integrated healthcare solutions that can seamlessly be introduced in healthcare systems;
- fostering development of safe and effective innovative health technologies and their combinations thanks to new and harmonised approaches to data generation;
- better and faster integration of future products, services and tools along the healthcare pathway (including health promotion and disease prevention), responding to patients’ specific needs and leading to improved health outcomes and patient well-being;
- patients and industry benefit from innovative manufacturing processes such as 3D printing, on-demand small-scale good manufacturing practice (GMP) synthesis, on-site portable production systems etc.;
- green transition enabled across all aspects of healthcare, both in the delivery of healthcare to patients, and in the technologies and products that emerge from a competitive European industry.
- b) contribute to strengthening the competitiveness of the EU’s health industry, via increased economic activity in the development of health technologies, in particular, integrated health solutions, thus fostering European technological leadership and the digital transformation of our societies.
Link: will be updated
Deadline: The call will be launched in early 2025; the deadline date will be provided once announced.
Indicative budget
Applicant consortia will be competing for the maximum financial contribution from IHI JU of up to EUR 30 000 000. NB: this amount is indicative and subject to change, pending approval by the IHI Governing Board.
IHI JU estimates that an IHI JU financial contribution of EUR 8 000 000 to 15 000 000 would allow a proposal to address these outcomes appropriately. NB: this amount is indicative and subject to change, pending approval by the IHI Governing Board. Nonetheless, this does not preclude the submission and selection of a proposal requesting different amounts
Scope:
With a view to harnessing new science and technologies, this topic aims to fund pre-competitive research and innovation for novel tools, methods, technologies etc. that will foster the development of health innovations to prevent, intercept, diagnose, treat, and manage diseases and enable recovery more efficiently.
Accordingly, applicants must assemble a collaborative public-private partnership consortium.
For example, proposals may aim to foster the development of integrated healthcare solutions, combining different technological areas and taking into account the needs of patients and citizens to, among others: a) facilitate patient contributions to R&I activities; b) support shared decision-making with healthcare professionals; and c) enable self-management of disease and health, de facto engaging in social innovation. This may imply, amongst others, the development of harmonised patient-reported outcome measures (PROMs) and patient-reported experience measures (PREMs), as well as the development of methods to elicit people’s preferences and digital tools to enable patient involvement.
Applicants should consider the following points in their proposals:
- a) address an unmet public health need based on at least one of the below:
- the high burden of the disease for patients and/or society due to its severity and/or the number of people affected by it;
- the high economic impact of the disease for patients and society;
- the transformational nature of the potential results on innovation processes where projects are not focussed on individual disease areas (e.g. health data analytics).
- b) have people-centric, rather than product- and pathology-centric, approaches – the focus being the patient and citizen journey through health care, with the help of most suitable health technologies and social innovations and taking account of demographic trends;
- c) demonstrate the ability to translate research into innovative solutions that can be integrated/implemented into the healthcare ecosystem and/or into industrial processes.
Expected impacts to be achieved by this topic
The actions to be funded under this topic are expected to achieve the following:
- a) contribute to one or more of the IHI JU’s expected impacts linked to the IHI JU’s Specific
Objective 3, as set out in the IHI JU SRIA, i.e.
- raised awareness among citizens and patients on their own role in managing their health;
- improved patient adherence to prevention programmes and medical interventions;
- people, including vulnerable populations (e.g. elderly and children as well as their carers and/or representatives), are better able to make informed decisions with their healthcare professionals about prevention, treatment interventions and disease management;
- increased frequency and quality of cooperation between patients, citizens and industrial stakeholders in the development of healthcare solutions, in particular integrated care solutions;
- patients benefit from prevention and treatment better adapted to their needs through improved diagnostic and monitoring;
- integrated healthcare solutions, including those based on the use of digital solutions, better responding to the needs and preferences of patients and citizens, supporting an inclusive approach;
- successful implementation of digital solutions supporting people-centred care;
- facilitated introduction of innovative solutions for improved home care of patients;
- healthcare solutions assessed according to criteria that matter to patients and citizens (in particular, PROMs and PREMs) contributing to achieving people-centred healthcare.
- b) contribute to strengthening the competitiveness of the EU’s health industry via increased economic activity in the development of health technologies, in particular, integrated health solutions, thus fostering European technological leadership and the digital transformation of our societies.
Link : will be updated
Deadline: The call will be launched in early 2025; the deadline date will be provided once announced.
Indicative budget
Applicant consortia will be competing for the maximum financial contribution from IHI JU of up to EUR 30 000 000. NB: this amount is indicative and subject to change, pending approval by the IHI Governing Board.
IHI JU estimates that an IHI JU financial contribution of EUR 8 000 000 to 15 000 000 would allow a proposal to address these outcomes appropriately. NB: this amount is indicative and subject to change, pending approval by the IHI Governing Board. Nonetheless, this does not preclude the submission and selection of a proposal requesting different amounts.
Scope:
With a view to harnessing new science and technologies, this topic aims to fund pre-competitive research and innovation for novel tools, methods, technologies etc. that will foster the development of health innovations to prevent, intercept, diagnose, treat, and manage diseases and enable recovery more efficiently.
Accordingly, applicants must assemble a collaborative public-private partnership consortium.
For example, proposals may aim at supporting the generation, pooling, integration and sharing of high- quality, harmonised, interoperable data (either existing or generated de novo), as well as the use of advanced analytical tools (including Artificial Intelligence, computational modelling and simulation or digital twin approaches). They may also support the development of better assistance systems for healthcare professionals to facilitate timely decision-making during disease course, thereby improving patient outcomes.
Applicants should consider the following points in their proposals:
- a) address an unmet public health need based on at least one of the below the high burden of the disease for patients and/or society due to its severity and/or the number of people affected by it;
- the high economic impact of the disease for patients and society;
- the transformational nature of the potential results on innovation processes where projects are not focussed on individual disease areas (e.g. health data analytics).
- b) demonstrate the ability to translate research into innovative solutions that can be integrated/implemented into the healthcare ecosystem and/or into industrial processes.
Expected impacts to be achieved by this topic
The actions to be funded under this topic are expected to achieve the following:
- a) contribute to one or more of the IHI JU’s expected impacts linked to the IHI JU’s Specific Objective 4, as reflected in the IHI JU SRIA, i.e.:
- wider availability of interoperable, quality data, respecting FAIR (findable, accessible, interoperable, reusable) principles, facilitating research and the development of integrated products and services;
- improved insight into the real-life behaviour and challenges of patients with complex, chronic diseases and co-morbidities thanks to m-health and e-health technologies;
- advanced analytics/artificial intelligence supporting health R&I, resulting in a) clinical decision support for increased accuracy of diagnosis and efficacy of treatment; b) shorter times to market; c) wider availability of personalised health interventions to end-users; d) better evidence of the added value from new digital health and artificial intelligence tools, including reduced risk of bias due to improved methodologies.
b) contribute to strengthening the competitiveness of the EU’s health industry via increased economic activity in the development of health technologies, in particular, integrated health solutions, thus fostering European technological leadership and the digital transformation of our societies.
Link : will be updated
Deadline: The call will be launched in early 2025; the deadline date will be provided once announced.
Indicative budget
Applicant consortia will be competing for the maximum financial contribution from IHI JU of up to EUR 20 000 000. NB: this amount is indicative and subject to change, pending approval by the IHI Governing Board.
IHI JU estimates that an IHI JU financial contribution of EUR 5 000 000 to 10 000 000 would allow a proposal to address these outcomes appropriately. NB: this amount is indicative and subject to change, pending approval by the IHI Governing Board. Nonetheless, this does not preclude the submission and selection of a proposal requesting different amounts.
Scope:
With a view to harnessing new science and technologies, this topic aims to fund pre-competitive research and innovation for novel tools, methods, technologies etc. that will foster the development of health innovations to prevent, intercept, diagnose, treat, and manage diseases and enable recovery more efficiently.
Accordingly, applicants must assemble a collaborative public-private partnership consortium.
For example, proposals may aim to develop methods and tools to assess the added value of emerging and converging health technologies, taking into consideration different stakeholders’ value dimensions, to support harmonised approaches for evidence generation.
Applicants should consider the following points in their proposals:
- a) address an unmet public health need based on at least one of the below:
- the high burden of the disease for patients and/or society due to its severity and/or the number of people affected by it;
- the high economic impact of the disease for patients and society;
- the transformational nature of the potential results on innovation processes where projects are not focussed on individual disease areas (e.g. health data analytics).
- b) demonstrate the ability to translate research into innovative solutions that can be integrated/implemented into the healthcare ecosystem and/or into industrial processes.
Expected impacts to be achieved by this topic
The actions to be funded under this topic are expected to achieve the following:
- a) contribute to one or more of the expected impacts linked to the IHI JU’s specific objective 5 as reflected in the IHI JU SRIA, i.e.:
- seamless and successful implementation in healthcare settings of cross-sectoral innovations, integrated products and services delivering proven benefits to patients, healthcare systems and society as a whole;
- patients have improved access to innovations that meet their needs and those of the healthcare systems;
- better informed decision-making at different levels of the healthcare system (authorities, organisations), that will in turn contribute to a better allocation of resources towards cost- effective innovations;
- faster entry to the market of cost-effective innovative solutions developed by industry, which could translate to a positive effect on their R&I investments
- b) contribute to strengthening the competitiveness of the EU’s health industry, via increase economic activity in the development of health technologies, in particular, integrated health solutions, and thus fostering European technological leadership and the digital transformation of our societies.

The aim of the call is to enable scientists in different countries to build an effective collaboration on a common interdisciplinary research project based on complementarities and sharing of expertise, with the expected impact being future use of the results to benefit patients. Projects will focus on a group of rare diseases or a single rare disease following the European definition
Topic List:
Research studies on therapies using small molecules, small non-coding chemically synthesized nucleic acid-based therapies, repurposed drugs or biologicals (e.g., antibodies or proteins such as enzymes, immune modulators or growth factors etc.). Proposals must cover at least one of the following areas:
- development of novel therapies in a preclinical setting through cell, organoid and animal model studies, molecule screening or use of in silico or artificial intelligence models
- development of predictive and pharmacodynamics biomarkers correlated to the efficiency of the therapy in a preclinical setting that could serve as surrogate endpoints
- replication of pre-clinical studies in an independent lab to increase validity of exploratory findings
- pre-clinical proof of concept studies for evidence of pharmacological activity in vitro and in vivo, pharmaco-kinetics and pharmaco-dynamics of the drug and first toxicology and safety data
- studies to support readiness for initiating clinical trial authorization conforming to regulatory requirements Translatability into humans should be the key focus of the project, and applicants should demonstrate access to relevant scientific or regulatory expertise (e.g., through innovation task forces or competent national authorities).
Excluded Approaches and Topics
The following approaches and topics are excluded from the scope of the JTC 2025:
- ATMP therapies (gene therapy medicinal product (including mRNA-based therapies), somatic cell therapy medicinal product, tissue engineered product, according to EMA definition).
- Development of new cell/organoid/animal models, which should already be established.
- Set-up or extension of natural history studies / patient registries.
- Interventional clinical trials to prove efficacy of drugs/treatments/surgical procedures/medical procedures. This includes studies comparing efficacy, e.g., two surgical techniques or therapies, and projects whose main objective is the implementation of a clinical phase IV pharmacovigilance study.
- Projects focusing only on rare neurodegenerative diseases that are within the focus of the Joint Programming Initiative on Neurodegenerative Disease Research (JPND). These are: Alzheimer’s disease and other dementias; Parkinson’s disease (PD) and PD-related disorders; Prion diseases; Motor Neuron Diseases; Huntington’s disease; Spinal Muscular Atrophy and dominant forms of Spinocerebellar Ataxia. Interested researchers should refer to the relevant JPND calls. However, childhood dementias/neurodegenerative diseases are eligible.
- Rare infectious diseases, rare cancers and rare adverse drug events in treatments of common diseases. Rare diseases with a predisposition to cancer are eligible.
The maximum duration of the project is three years.
Budget: Application budgets are not limited but need to reflect the actual needs of the proposed project.
Deadline: February 05, 2025
Eligibility: Non-domestic (non-U.S.) Entities (Foreign Organizations) are eligible to apply.
Non-domestic (non-U.S.) components of U.S. Organizations are eligible to apply.
Foreign components, as defined in the NIH Grants Policy Statement, are allowed.
Purpose of this funding opportunity announcement is to encourage collaborations- between life science and physical science- that: 1) apply a multidisciplinary bioengineering approach to solve biomedical problems; and 2) develop, integrate, optimize, validate, translate or accelerate adoption of promising tools, methods and techniques: a) that fulfill an unmet need and address specific research or clinical problem in basic, translational, and/or clinical science and practice, b) capable of enhancing our understanding of health and disease, and/or c) improve practice of medicine. Applications may propose design-directed, developmental, discovery-driven, or hypothesis-driven research, and this FOA is appropriate for small teams applying an integrative approach to increase our understanding of and solve problems in biological, clinical or translational science.
Research Objectives
Many major biomedical research problems are best addressed with a multidisciplinary approach that bridges life sciences and physical sciences. Principles and techniques in quantitative sciences such as physics, mathematics, chemistry, computer sciences, and engineering are increasingly applied to enable and advance biomedical research. Bioengineering approaches integrate principles from diverse technical and biomedical fields, and the resulting multi-disciplinary research provides new understanding, innovative technologies, and new products that improve basic knowledge, human health, and quality of life. This FOA seeks to encourage collaborations among investigators in the fields of quantitative science and physical science with biomedical researchers to catalyze the development of innovative bioengineering approaches to solve important problems in biomedical research, translational research, clinical investigations, and medical practice.
Significant projects may include, but are not limited to: development, validation, and translation of promising modalities for the disease continuum, including tools for risk prediction, screening, prevention, detection, diagnosis, disease progression, intervention, monitoring treatment response, prognosis, or survival; development of quantitative, predictive models of complex biological systems; integration and optimization of technologies that significantly increase sensitivity, specificity, positive predictive value, negative predictive value, efficiency, or throughput of analysis to address unsolved biological or medical questions; in vitro and in vivo models, cell/tissue culture systems and organoids that closely mimic physiological conditions and allow mechanistic studies or engineering and testing of delivery systems, molecules/cells/tissues for therapeutic purposes, therapeutics, implants, and prosthetics that may improve treatment and healthcare.
Innovation in this biomedical engineering FOA has a broad definition that includes development of new methods, ideas, or tools, integration of existing components into new combinations that deliver new and/or greater capabilities, new efficiencies, and/or greater effects. Overall impact of these advances may include improving our understanding of molecular mechanisms, reducing disparities in access to care, promoting wellness and independent living, increasing access to and utility of technologies to improve quality of life, reducing the cost and complexity of procedures, and/or increasing throughput, sensitivity, and specificity of diagnostic tests.
Applications Not Responsive to this FOA
The following types of studies are not responsive to this FOA. Applications proposing such studies will be considered non-responsive and will not be reviewed:
Applications that do not seek to demonstrate feasibility and/or potential utility of new capabilities or improvements for solution of problems in basic biomedical, pre-clinical, or clinical research, clinical care delivery, or accessibility will be considered nonresponsive to this funding opportunity announcement, for example:
- Pursuit of a biological or clinical hypothesis where no technology development is proposed;
- Using existing technologies that do not need modifications or adaptations to answer a biomedical question; and
Traditional biological hypothesis-driven research using unmodified existing technologies.
Budget: The number of awards is dependent upon NIH budgets and the submission of a sufficient number of deserving applications. Application budgets are not limited but need to reflect the actual needs of the proposed project. The scope of the proposed project should determine the project period.
Deadline: February 05, 2025
Eligibility: Non-domestic (non-U.S.) Entities (Foreign Organizations) are eligible to apply. Non-domestic (non-U.S.) components of U.S. Organizations are eligible to apply. Foreign components, as defined in the NIH Grants Policy Statement, are allowed.
The purpose of this funding opportunity announcement (FOA) is to encourage collaborations between the life and physical sciences that: 1) apply a multidisciplinary bioengineering approach to the solution of a biomedical problem; and 2) integrate, optimize, validate, translate or otherwise accelerate the adoption of promising tools, methods, and techniques for a specific research or clinical problem in basic, translational, or clinical science and practice. An application may propose design-directed, developmental, discovery-driven, or hypothesis-driven research and is appropriate for small teams applying an integrative approach to increase our understanding of and solve problems in biological, clinical, or translational science.
This FOA will support clinical trials that test functionality or validate performance in the chosen setting. This FOA is not intended to support conventional clinical trials that lack translation as the primary motivation. Applications that propose phase III clinical trials in any area of research are not sought by and will not be supported through this FOA. This FOA does not propose to support commercial production
Research Objectives
Many major biomedical research problems are best addressed with a multidisciplinary approach that bridges the life and physical sciences. Principles and techniques in quantitative sciences such as physics, mathematics, chemistry, computer sciences, and engineering are increasingly applied to good effect in biomedical research. Bioengineering approaches integrate principles from diverse technical and biomedical fields, and the resulting multi-disciplinary research provides new understanding, innovative technologies, and new products that improve basic knowledge, human health, and quality of life. This FOA seeks to encourage collaborations of quantitative and physical scientists with biomedical researchers to catalyze the development of innovative bioengineering approaches to the solution of important problems in biomedical research, clinical investigations, and medical practice.
Significant projects may include, but are not limited to: validation and translation of promising tools for prevention, monitoring or intervention; development of quantitative, predictive models of complex biological systems; integration and optimization of technologies that significantly increase sensitivity, specificity, positive predictive value, negative predictive value, efficiency, or throughput of measurements to address unsolved biological or medical questions; or engineering and testing of delivery systems, tissues, therapeutics, implants, and prosthetics that may improve treatment and healthcare.
Innovation in this biomedical engineering FOA has a broad definition that includes development of new methods, ideas, or tools, integration of existing components into new combinations that deliver greater capabilities, new efficiencies, and/or greater effects. Overall impact of these advances may include reducing disparities in care, promoting wellness and independent living, increasing access to and utility of technologies to improve quality of life, reducing cost and complexity of procedures, and increasing throughput, sensitivity, and specificity of diagnostic tests.
This FOA will support clinical trials that test functionality or validate performance in the chosen setting. This FOA is not intended to support conventional clinical trials that lack translation as the primary motivation. Applications that propose phase III clinical trials in any area of research are not sought by and will not be supported through this FOA. This FOA does not propose to support commercial production.
Applications Not Responsive to this FOA
The following types of studies are not responsive to this FOA. Applications proposing such studies will be considered non-responsive and will not be reviewed:
- Late phase or randomized clinical trials (although studies using specimens from these trials are appropriate);
- Applications that propose phase III clinical trials in any area of research are not responsive to this FOA.
The CURE Epilepsy Catalyst Award (2 years / $250,000) supports nimble development of data necessary to advance ideas toward larger commercialization funding opportunities and is not intended to replace those opportunities.
Requests may be made for up to a total of $250,000 paid over 2 years. Funding requests may include salary support for the PI, technical staff and/or collaborators, supplies, animal costs, etc., and travel to an epilepsy-related conference if the PI is presenting his/her CURE Epilepsy-funded research. Limited equipment purchases that are required to complete goals will be considered. Indirect costs are not supported.

The CURE Epilepsy Award (2 years / $250,000) reflects CURE Epilepsy’s continued focus on scientific advances that have the potential to truly transform the lives of those affected by epilepsy, with prevention and disease modification as critical goals. Key priority areas for the award include:
- Basic mechanisms of epilepsy
- Acquired epilepsies
- Pediatric epilepsies
- SUDEP
- Treatment-resistant epilepsies
- Sleep & epilepsy
This award is available to both established and early career investigators*. Researchers who serve on CURE Epilepsy’s Scientific Advisory Council are ineligible to apply for or sponsor a grant for the duration of their term. International applicants are welcome.
Requests may be made for up to a total of $250,000 paid over 2 years. Funding requests may include salary support for the PI, technical staff and/or collaborators; supplies, animal costs, etc.; and travel to an epilepsy-related conference if the PI is presenting his/her CURE Epilepsy-funded research. Limited equipment purchases that are required to complete goals will be considered. Indirect costs are not supported.
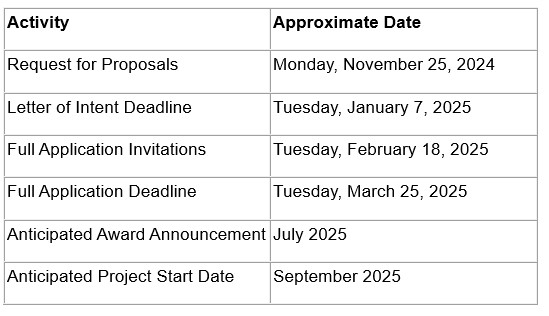
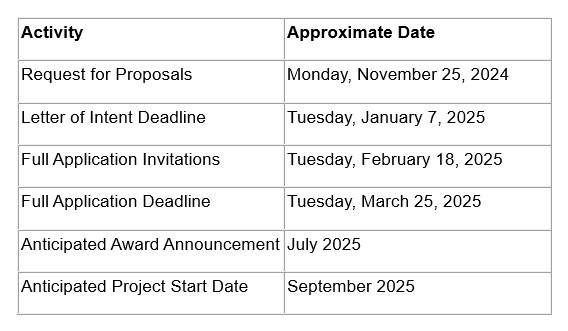
The Rare Epilepsy Partnership Award (1 year / $100,000) will support the development of necessary research tools, techniques, model systems, and data collection platforms to stimulate and accelerate research on rare epilepsies. Each award will be co-funded by CURE Epilepsy and one or more of the rare epilepsy advocacy groups (partners) identified in the Request For Proposals. Applications must focus on one or more of the specific rare epilepsies that are represented by each group as well as address CURE Epilepsy’s mission to cure epilepsy.
Budget : Funding requests must be itemized and based on specific, milestone-defined scientific aims. Requestsmay be made for up to a maximum of $100,000 paid over one year. CURE Epilepsy reserves the right to fund only select specific aims or stage funding of proposals based on the achievement of milestones. Budgets may include salary support for the Principal Investigator (PI), technical staff and/or co-PIs, supplies, animal costs, vendor costs, limited equipment costs, and travel to an epilepsy-related conference only if the PI is presenting his/her CURE Epilepsy-funded research. Indirect costs are not supported.
This award is available to both established and early-career investigators. Established investigators are university faculty at the associate professor level or above, or investigators who hold an equivalent position in a non-university research organization. Early career investigators are defined as a) university faculty at the assistant professor level or hold an equivalent position in a non-university research organization, b) researchers with an appointment as an instructor or research assistant professor, c) post-doctoral fellows with at least three years of post-doctoral experience or d) clinical fellows. Early career investigators must have a mentor committed to advising the applicant.

Deadline: 21 May 2025
Grant funding: Pathfinder Open – grants of up to EUR 3 million or more if duly justified Pathfinder projects can also receive additional funding for testing the innovation potential of their research outputs.
Do you have an ambitious vision for a novel future technology that could make a real difference to our lives?
- Do you see a plausible way of achieving the scientific breakthrough that will
make this technology possible?
- Can you imagine collaborating with an interdisciplinary team of researchers and innovators to validate the scientific basis of the future technology, realise a proof of principle, and explore paths to impact?
If the answer to each one of these questions is ‘yes’, then EIC Pathfinder Open may be the right call for you.
The EIC Pathfinder is a funding programme under Horizon Europe that offers support to research teams by:
- funding research to develop the scientific basis to underpin breakthrough technologies
- supporting the earliest stages of scientific, technological or deep-tech R&D
- aiming to build on new, cutting-edge directions in science and technology to disrupt a field and a market or create new opportunities
- realising innovative technological solutions to identify, develop and scale up breakthrough technologies and disruptive innovations in Europe
‘EIC Pathfinder Open’ open to support projects in any field of science, technology or application without predefined thematic priorities.
Budget: between $25,000 and $75,000
The Alliance’s next Research Grant Cycle will open November 1, 2024
The goal of the PCDH19 Alliance Research Grant Program is to fund research directly towards understanding the expression of the PCDH19 gene and the function of the PCDH19 protein, finding therapeutic treatments, and a cure for PCDH19 Epilepsy.
We also hope that the projects we fund will lead to additional research support from government or other funding agencies. We are pleased to be able to support many different types of projects, each critical for advancing all phases of PCDH19 research, from basic to clinical to treatment.
These grants are crucial for allowing investigators to gain enough data to be able to attract even larger, multi-year commitments from the National Institutes of Health (NIH) and other traditional medical research funding organizations. The PCDH19 Alliance Research Grant Program awards one year grants between $25,000 and $75,000. The number of awards is contingent upon the availability of funds for that cycle.
Submission deadline for pre-proposals: 30 January 2025, 14:00 CET
Submission deadline for full-proposals: 19 June 2025, 14:00 CEST
The Transforming Health and Care Systems (THCS) initiative, established as a European Partnership under Horizon Europe and co-funded by the European Commission, aims to address the increasing burdens on European health and care systems. The initiative focuses on developing coordinated, harmonized solutions involving EU member states, associated countries, research and innovation funders, and other public authorities. The overarching goal is to ensure high-quality, sustainable health and care services by fostering transnational collaboration, aligning regional and national research strategies, and promoting interdisciplinary excellence.
Aim of the call
The aim of this call is to fund research and innovation projects that strengthen primary and community health and care systems and provide policy and decision makers with the necessary knowledge and tools to govern the transitions needed in the primary andcommunity care sector. Projects funded under this call will deliver promising financial, organizational, and practice-based service innovations that promote the transformation of health and care systems and contribute to faster exchange of best practices across different countries and regions.
Proposals are expected to address one of two sub-topics:
- Sub-topic 1: Strengthening the primary and community health and care system – This involves reducing reliance on institutionalized treatment in favour of seamless care pathways and different forms of primary and community care through organizational innovations, operational improvements, and innovative models of service delivery.
- Sub-topic 2: Systemic approaches to modernizing the primary and community care sector – This involves providing evidence-based knowledge to support policy development and strategic planning for the modernization of the primary and community care sector, leveraging multidisciplinary and transnational perspectives.
Travel & Mobility Grants, Doctoral programs
Budget: $500
The application process is very straightforward and quick. There are no complicated rules or terms and conditions – you just need to be a post-graduate attending a relevant conference, and be able to tell us why you think you deserve it. However, to make it fair to all, there are just a few requirements:
- you need to be a current post-graduate
- you must provide a detailed breakdown of what the money will be used for, and what funding (if any) you have already obtained. Things we are looking for are direct travel costs, or registration fees – not your beer supply for the week!
- and finally – after you have submitted your application, we will need a short statement from your supervisor / PI to confirm your application.
The Boehringer Ingelheim Fonds (BIF) awards travel grants of up to three months duration to MD and PhD students, as well as postdoctoral researchers from all over the world. The BIF supports them if they conduct experimental projects in basic biomedical research and want to pursue short-term research stays or attend practical courses relevant to their projects in Europe or overseas.
The programme also enables graduate students and their potential supervisors to evaluate the scientific and personal fit before starting a PhD project abroad.
They support junior scientists who want to learn clearly-defined methods useful for their ongoing research and their current laboratory by
- Visiting another laboratory
- Attending research-orientated courses with the practical part making up at least 50 % of the course.
They can also be used by PhD candidates and their potential supervisors to evaluate the scientific and personal fit before the start of a PhD project in another country by funding a research stay of one to three months in the supervisor’s laboratory.
EMBO Scientific Exchange Grants fund research exchanges of up to three months between laboratories in eligible countries. The grants facilitate collaborations with research groups with expertise, techniques, or infrastructure that is unavailable in the applicant’s laboratory. They cover travel and subsistence costs of the fellow.
Duration of support
The grants are intended for visits of one week (seven days) up to three months (90 days). Awarded applicants can stay on their research visit for an additional three months (maximum), however, EMBO will not provide funding for this extended period. It is possible to apply directly for more than three months (up to six), but the EMBO grant must be used for the first three months of the visit.
Deadline model: single-stage
Planned opening date: 29 May 2024
Deadline date: 27 November 2024 17:00:00 Brussels time
Budget: €451 153 000
Expected Outcome:
Project results are expected to contribute to the following outcomes:
For supported doctoral candidates
- New research and transferable skills and competences, leading to improved employability and career prospects within and outside academia;
- New knowledge allowing the conversion of ideas into products and services, where relevant;
- Enhanced networking and communication capacities with scientific peers, as well as with the general public that will increase and broaden the research and innovation impact.
For participating organisations
- Improved quality, relevance and sustainability of doctoral training programmes and supervision arrangements;
- Enhanced cooperation and transfer of knowledge between sectors and disciplines;
- Increased integration of training and research activities between participating organisations;
- Boosted R&I capacity;
- Increased internationalisation and attractiveness;
- Regular feedback of research results into teaching and education at participating organisations.
Scope:
MSCA Doctoral Networks will implement doctoral programmes, by partnerships of universities, research institutions and research infrastructures, businesses including SMEs, and other socio-economic actors from different countries across Europe and beyond. MSCA Doctoral Networks are indeed open to the participation of organisations from third countries, in view of fostering strategic international partnerships for the training and exchange of researchers.
These doctoral programmes will respond to well-identified needs in various R&I areas, expose the researchers to the academic and non-academic sectors, and offer training in research-related, as well as transferable skills[1] and competences relevant for innovation and long-term employability (e.g. entrepreneurship, commercialisation of results, Intellectual Property Rights, communication). Proposals for doctoral networks can reflect existing or planned research partnerships among the participating organisations.
The selection procedure for doctoral candidates must be open, transparent and merit-based, in line with the Code of Conduct for the Recruitment of Researchers. The vacancy notice (to be widely advertised internationally, including on the EURAXESS[2] website) must mention if the published rates include all employer and employee’s taxes and contributions. If possible, the gross salary (net salary + employee’s taxes and contributions) should be published.
MSCA Doctoral Networks are encouraged to lead to Industrial or Joint Doctorates.
Training activities
MSCA Doctoral Networks should exploit complementarities between participating organisations and foster sharing of knowledge and networking activities for example through the organisation of workshops and conferences. Proposed training activities should respond to well identified needs in various R&I areas, with appropriate references to inter- and multidisciplinary fields and follow the EU Principles for Innovative Doctoral Training[4]. They should be primarily focused on developing new scientific knowledge through original research on personalised projects.
Deadline model: single-stage
Planned opening date: 10 October 2024
Deadline date: 05 February 2025 17:00:00 Brussels time
Budget: 81 226 000
ExpectedOutcome:
Project results are expected to contribute to the following outcomes:
For staff members
Increased set of research and transferable skills and competences, leading to improved employability and career prospects within and outside academia;
More knowledge and innovative ideas converted into products, processes and services;
More entrepreneurial mind-sets, testing new and innovative ideas;
Increased international exposure leading to extended networks and opportunities;
Enhanced networking and communication capacities with scientific peers, as well as with the general public that will increase and broaden the research and innovation impact.
For participating organisations
Innovative ways of cooperation and transfer of knowledge between sectors and disciplines;
Strengthened and broader international, inter-sectoral and interdisciplinary collaborative networks;
Boosted R&I capacity.
Scope:
MSCA Staff Exchanges involve organisations from the academic and non-academic sectors (including SMEs) from across the globe.
Support is provided for international, inter-sectoral and interdisciplinary mobility of R&I staff leading to knowledge transfer between participating organisations.
Two programs :
- HORIZON-TMA-MSCA-Cofund-D HORIZON TMA MSCA Cofund Doctoral programme
- HORIZON-TMA-MSCA-Cofund-P HORIZON TMA MSCA Cofund Postdoctoral programme
Deadline model: single-stage
Planned opening date: 08 October 2024
Deadline date: 06 February 2025 17:00:00 Brussels time
Budget: €99 276 000
Expected Outcome:
Projects results are expected to contribute to the following outcomes:
For supported doctoral candidates or postdoctoral researchers
Deeper and more diverse set of research-related and transferable skills and competences;
Improved employability and career prospects both within academia and beyond;
New mind-sets and approaches to R&I work forged through international, inter-sectoral and interdisciplinary experience;
Enhanced networking and communication capacities with scientific peers, as well as with the general public that will increase and broaden the research and innovation impact.
For participating organisations
Enhanced quality and sustainability of research training;
Increased global attractiveness, visibility and reputation of the participating organisation(s);
Stronger R&I capacity and output among participating organisations;
Increased contribution of the participating organisations to the local, regional and/or national socio-economic ecosystems;
Regular feedback of research results into teaching and education at participating organisations.
Scope:
Applicants submit proposals for new or existing doctoral or postdoctoral programmes with an impact on the enhancement of human resources in R&I at regional, national or international level. These programmes will be co-funded by MSCA COFUND.
Proposed programmes can cover any research disciplines (“bottom-up”), but exceptionally can also focus on specific disciplines, notably when they are based on national or regional Research and Innovation Strategies for Smart Specialisation (RIS3 strategies). In this case, the range of covered disciplines should allow reasonable flexibility for the researchers to define their topic.
Funding synergies with Cohesion policy funds and the Recovery and Resilience Facility (RRF) are strongly encouraged
A Career Development Plan must be jointly established by the supervisor and each recruited researcher upon recruitment. In addition to research objectives, this Plan comprises the researcher’s training and career needs, including training on transferable skills, teaching, planning for publications and participation in conferences and events aimed at opening science and research to citizens. The Plan must be established at the beginning of the recruitment and should be revised (and updated where needed) within 18 months.
COFUND takes the form of:
A) Doctoral programmes
Doctoral programmes offer research training activities to allow doctoral candidates to develop and broaden their skills and competences. They will lead to the award of a doctoral degree in at least one EU Member State or Horizon Europe Associated Country.
Substantial training modules, including digital ones, addressing key transferable skills and competences common to all fields, fostering good scientific conduct such as research integrity, and fostering the culture of Open Science, innovation and entrepreneurship will be supported.
On top of compulsory international mobility, applicants are encouraged to include elements of cross-sectoral mobility and interdisciplinarity into their programmes. Collaboration with a wider set of associated partners, including from the non-academic sector, will be positively taken into account during the evaluation. These organisations may provide hosting or secondment opportunities or training modules in research or transferable skills.
Particular attention is paid to the quality of supervision and mentoring arrangements as well as career guidance. The selection procedure for doctoral candidates must be open, transparent and merit-based, in line with the Code of Conduct for the Recruitment of Researchers.
B) Postdoctoral Programmes
Postdoctoral Programmes fund individual advanced research training and career development fellowships for postdoctoral researchers. The programmes should offer training to develop key transferable skills and competences common to all fields, foster good scientific conduct such as research integrity, foster innovation and entrepreneurship and promote and (where appropriate) reward Open Science practices (open access to publications and to other research outputs including data, FAIR data management, societal engagement and citizen science, etc.).
Postdoctoral Programmes should have regular selection rounds following fixed deadlines or regular cut-off dates, allowing fair competition between researchers. The selection procedure for postdoctoral candidates must be open, competitive, merit-based and with a transparent international peer review, in line with the Code of Conduct for the Recruitment of Researchers.
The Taking Flight Award (1.5 years / $125,000) seeks to promote the careers of early-career investigators to allow them to develop an independent research focus.
You must fall into one of the following categories to be eligible for the Taking Flight Award:
- A postdoctoral fellow with a PhD, PsyD, PharmD, or equivalent and a minimum of two years postdoctoral experience at the time of submission
- A clinical fellow who is a Neurology Resident in his/her Neurology training and considering Epilepsy Fellowships
- Newly appointed faculty within one year of having completed postdoctoral training
- Clinician-researchers who are within two years of their faculty appointment
International applicants are welcome; you do not have to be a US citizen or working in the US to apply for this award. All materials must be submitted in English.
Requests may be made for up to $125,000 for eighteen months. Funding requests may include salary support for the PI, technical staff and/or collaborators; supplies, animal costs, publication fees etc.; and travel to an epilepsy-related conference if the PI is presenting his/her CURE Epilepsy-funded research. Funds are not to be used to purchase equipment. Indirect costs are not supported.
More details coming soon…
The Career Development Commission and its Fellowships Task Force will open the call for applications for the ILAE-Europe visiting scholarship scheme in September 2024. This funding opportunity will support eligible early-career scientists and clinicians undertaking a training visit to a prestigious epilepsy center in Europe.
Other (Prize, Networking, Infrastructure, workshop funding…)
Deadline: 21 October 2025 (12:00 CEST). Applications can be submitted at any time until the next Collection Date deadline.
Budget: No budget forecast is requested when submitting a proposal. An estimated €125,000 is made available for a COST Action in its first year and an average of €150,000 per year for the other three years. The amounts are variable from a grant period to another and depends, among others, on the size of the network and overall budget available to COST. The funders anticipate supporting up to 70 new COST Actions in the 2025 cycle.
Link: https://www.cost.eu/
COST (European Cooperation in Science and Technology) is an intergovernmental framework for European Cooperation in the field of scientific and technical research. COST does not fund research itself, but funds the expenses of interdisciplinary research networks called COST Actions. These support a range of networking tools, such as workshops, conferences, training schools, short-term scientific missions (STSMs), communication activities, and virtual networking tools.
COST Actions enable researchers from academia, SMEs, public institutions and other relevant organisations to investigate a topic of their choice for four years. They are open to all science and technology fields, including new and emerging fields, and offer an inclusive, pan-European environment for individuals of all levels of seniority to grow their professional research networks and boost their careers.
Proposals for new Actions are bottom-up and can be on any topic. Researchers can participate by submitting a proposal for a new COST Action, or by joining an existing COST Action. COST Actions are open throughout their lifetime to new members including researchers, engineers and scholars or other stakeholders.
Participation is open to researchers in universities, research centres, and large and small public and private organisations from all COST Member Countries and its Cooperating State (Israel) and Partner Member (South Africa).
Proposals for COST Actions must include a Network of Proposers from at least seven different COST Full or Cooperating Members amongst which a minimum of 50% must be from COST Inclusiveness Target Countries.
COST Member Countries: Albania, Armenia, Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Republic of Moldova, Montenegro, Netherlands, Republic of North Macedonia, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, Ukraine and United Kingdom.
Inclusiveness Target Countries: Albania, Armenia, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Estonia, Georgia, Greece, Hungary, Latvia, Lithuania, Malta, Moldova, Montenegro, Poland, Portugal, Romania, Slovakia, Slovenia, North Macedonia, Serbia, Türkiye and Ukraine.
The funding a COST Action receives covers the expenses of networking activities rather than research. Financial support is available for four years to cover coordination costs such as contributions to workshops/conferences, travel costs for meetings, contributions to publications and short-term scientific missions of researchers to visit other laboratories.
Budget: € 15000
Deadline for application: December 31st, 2024
Stiftung Michael (SM – Michael Foundation) supports scientific research into the causes of seizure disorders and the most appropriate methods of treating them, while also combating their individual and social consequences in Germany.
The biennial Michael Prize (Michael-Preis) is designed to promote epilepsy research and honour outstanding scientific contributions which have furthered epileptology.
Eligible to apply are researchers worldwide who have not yet reached the age of 45 years at the time of application deadline.
The competition is open for the categories
- Neurology, neuropediatrics, neurosurgery
- Public health, social sciences
- Imaging.
Each category of the award is endowed with €15,000.
The following papers/documents are required
- Proof of age: not yet reached the age of 45 on December 31st, 2024,
- up to three scientific papers in English language which are published or accepted for publication
- for papers accepted for publication a copy of the acceptance letter must be submitted
- at least one of the papers must be from the period 2023 – 2024
- an indication which of the three categories the applicant’s research is referring to
- a curriculum vitae.
The submitted applications will be rated by an independent jury consisting of:
- Eleonora Aronica, Amsterdam (The Netherlands)
- Yushi Inoue, Shizuoka (Japan)
- Jean Gotman, Montreal (Canada)
Deadline date: 14 January 2025 17:00:00 Brussels time
Budget : €1 500 000
Project results are expected to contribute to the following outcomes:
- A more consistent and sustained level of coordination and preparedness for supporting researchers at risk at European, national and institutional level;
- Improved support to researchers at risk through the provision of policy recommendations, as well as advice and assistance on their implementation;
- A more sustainable and professionalised support network/structure/system for researchers at risk across Europe, facilitating access to funding and networking opportunities, creating level playing field for applicants to European and national R&I programmes, and raising the quality of submitted proposals;
- More synergies between initiatives supporting researchers at risk funded by EU programmes (such as Horizon Europe and Erasmus+) and national or institutional actors;
- Increased exposure of researchers at risk to the industry and to the non-academic sector;
- Greater awareness in Europe and beyond on why researchers are at risk and ways to support them.
Scope:
To build on the available results of past and on-going Researchers at Risk initiatives[1] further support is envisaged towards national and international organisations working with researchers at risk and aiming to enhance and professionalise their activities. It should further facilitate and strengthen cooperation and linkages between European, national and institutional initiatives and programmes, increasing awareness on why researchers are at risk, as well as identifying and delivering the best possible solutions to the challenges these researchers are confronted with.
The support action should be aligned with the general objectives of the MSCA, in particular scientific excellence, skills and career development, inter-sectoral mobility, equal opportunities and inclusiveness, attractive working conditions, work/life balance, while fostering open science, innovation and entrepreneurship. It should not duplicate other actions foreseen under Horizon Europe or other EU-funded programmes such as Erasmus+, but rather build synergies between these programmes. The activities carried out under this support action should complement actions in Member States and third countries associated to Horizon Europe.
The expected duration of the action is 36 months.
Funding for scientific meetings or workshops relevant to neurobiology of epilepsy can also be provided through the Neurobiology Commission. Requests for sponsoring such workshops are considered for funding on an annual basis by the Neurobiology Commission. To apply, please submit the Neurobiology Commission Funding Request Form to nbcbursaries@ilae.org by 31 July of the year preceding the planned meeting. Decisions on support will be announced by the end of March of the year of the event.
You can sign up below to receive the EpiCARE Research Calls Newsletters, sent a few times a year, to inform about the new research calls:

