EpiCARE Working Groups
EpiCARE’s core programme is detailed in a work plan, work packages and delivrables. They are providing a detailed project plan, the timing of the work packages and their description.
You can find out more about EpiCARE’s core programme exploring the different work packages below (click on the + sign to develop the section)

Best practices in epilepsy care
Summary
Increasingly genetic, autoimmune and metabolic epilepsies are being recognised although few characteristic epilepsy phenotypes have been identified (i.e. PCDH19, SCN1A, NMDA encephalitis, GLUT1 deficiency) that permit prediction of which aetiology is likely involved. Progressive refinement of the phenotype will reduce the number of patients who require molecular genetics, for diagnostic purposes (with consequent financial impact, particularly for patients with poor social coverage). Neural autoantibody testing and metabolic testing can be also more targeted. Refining phenotypes requires that specific features (seizure, EEG, MRI characteristics, cognitive profile, psychiatric, movement and other possible comorbidities,) are identified accurately by physicians with appropriate expertise. Knowledge of these additional symptoms and their prediction may permit actions to reduce their impact. For given conditions, few experts have devoted time on the identification of the phenotype. Experts need to be identified from within the network so that they may contribute to this phenotypic refinement. The identification of aetiological groups throughout the EU will be able to provide appropriate genetic, metabolic and immune testing to a wider population.
Leaders
Objectives
- To refine the clinical/EEG phenotype with genotype correlation (characteristics of the epilepsy, including age and mode of onset and course, type of cognitive disorders, minimum features necessary for the diagnosis – in which cases genotyping is mandatory)
- To optimize the genetic and biochemical work-up to save resources when possible, and complete with functional validation if needed
- Identify groups providing next generation sequencing and new gene identification
- Identify expert groups able to provide genetic evaluation and diagnosis
- To refine the clinical/EEG phenotype with autoimmune aetiology correlation (characteristics of the epilepsy, including age and mode of onset and course, type of comorbid disorders)
- Identify expert groups able to provide neural antibody testing/autoimmune disease evaluation and diagnosis
Neuroimaging
Neuroimaging investigations are fundamental in the diagnosis of patients with epilepsy, in identification of etiology of epilepsy and in selected cases for surgical planning. In recent years, there have been tremendous advances in the availability and acquisition of structural MRI, functional MRI, PET and SPECT imaging. Furthermore, advanced post-processing methods and machine-learning algorithms have been applied to neuroimaging data in patients with epilepsy. This working group aims to promote harmonisation of best practices in neuroimaging evaluation of rare and complex epilepsies to enable and ensure state-of-the-art care across EpiCARE. We have a group of radiologists and neuroimaging post-processing experts from across the EpiCARE network who meet to share best practice and create recommendations. We also organise EpiCARE webinars on neuroimaging in rare and complex epilepsies. We are currently planning post-processing workshops to improve the technical neuroimaging skills of EU professionals involved in the management of rare and complex epilepsies. This will facilitate the use of state-of-the-art neuroimaging post-processing tools as research adjuncts for surgical evaluation of epilepsy patients as well as facilitating the participation of EU centres in multi-centre research projects (such as ENIGMA -Epilepsy and the MELD Project).
Please find here the results of the Neuroimaging survey on MRI protocols and other neuroimaging methods in EpiCARE centres, and the Summary of recommendations for neuroimaging in epilepsy in general, and in specific patient populations.
Leaders
Neuropsychology
Summary
Behavioural and cognitive comorbidities in epilepsy are very common. They are often present already at epilepsy onset and before. Cognitive and behavioural assessments can reveal differential diagnostic indicators of epilepsy subtypes and syndromes, particularly when related to data from imaging, electrophysiology, pathology, and genetics (see other work packages). Furthermore it allows monitoring of disease dynamics (progress and control), and it can be used for the outcome and quality control of applied treatments (surgical, stimulatory, medical, behavioural comprehensive care). With the E-Neuropsych task force within the funded pilot ERN E-pilepsy an initiative was taken which aimed at the homogenization and distribution of best evidence based neuropsychological practice. The result was the availability of a core standard test battery of evidence based neuropsychological and behavioural instruments. The battery covers developmental and adult neuropsychology and addresses various cognitive and behavioural domains. The tool box was implemented in a web based E-Neuropsych platform for data entry, evaluation, collection and communication across EU reference centres for epilepsy surgery. E-Neuropsych represents one node within a diagnostic network which also comprises imaging (structural/functional), and electrophysiological (invasive/non-invasive) diagnostics and evaluation tools. E-Neuropsych, can be extended and adapted to all other questions concerning the epilepsies. Within a large network of clinics it can well be expected that for rare pathologies enough data can be collected so that their cognitive and behavioural aspects along with disease development and treatment can be defined.
Leaders

Pr. Christoph Helmstaedter
Bonn, Germany

Dr. Andrea Palacio
Barcelona, Spain

Dr. Anna Lopez
Barcelona, Spain

Dr. Julia Taube
Bonn, Germany
Objectives
- Set up of a common evidence based diagnostic toolbox for assessing and monitoring behavioural comorbidities of the epilepsies
- Set up of a registry for cognitive and behavioural comorbidities in epilepsy across European epilepsy centres
- Discerning differential diagnostic and prognostic criteria for different disease conditions in epilepsy from the beginning of the disease and along its natural course
- Outcome and quality control of different treatment options
- Development of new therapeutic or prophylactic approaches to behavioural comorbidities in epilepsy
Neurophysiology
Summary
Clinical Neurophysiology methods, especially EEG, provide valuable information for diagnosing the epilepsies, with specific patterns identified in many diseases requiring expert interpretation. Thus, integration of recent advances into EpiCARE is essential. However experience and expertise is required to recognise the advantage of accurate recording and interpretation; geographical variation requires specifically to be addressed here.
SCORE is a computer-based system for EEG assessment and reporting, which reduces the inter-observer agreement in EEG interpretation and is based on a consensus proposal by a group of European experts endorsed by the International League Against Epilepsy – Commission on European Affairs (ILAE-CEA) and the European Chapter of the International Federation of Clinical Neurophysiology (EC-IFCN). During the development process, SCORE was tested and adjusted by applying it to real, clinical data.
Electrical source imaging is part of the presurgical evaluation of patients with drug-resistant focal epilepsy. The software packages that will be used in this study have CE mark for this specific medical use. In spite of being part of the clinical standard, the evidence for the accuracy and clinical utility of these methods are derived from several smaller-scale and retrospective studies. The PROMAESIS study will provide solid evidence of the accuracy and clinical utility of automated ESI.
Publications
- Beniczky S, Aurlien H, Franceschetti S, et al. Interrater agreement of classification of photoparoxysmal electroencephalographic response. Epilepsia. 2020;61(9):e124-e128. doi:10.1111/epi.16655
- Krysl D, Beniczky S, Franceschetti S, Arzimanoglou A. The COVID-19 outbreak and approaches to performing EEG in Europe. Epileptic Disord. 2020;22(5):548-554. doi:10.1684/epd.2020.1208
- Clinical Utility of Automated Electric Source Imaging in Presurgical Evaluation (PROMAESIS)
- D4.1. Report on availability and standard of EEG investigations across centres in EpiCARE
Leaders
Objectives
- Development of a web-based platform for secure exchange and storage of neurophysiological data, integrated with the databases of other work-packages
- Integration of SCORE into EpiCARE
- Validation of automated EEG source imaging in presurgical evaluation of patients with drug resistant focal epilepsy
Neuropathology
Summary
Microscopic tissue diagnosis of human brain disorders remains the gold standard in modern medicine. Yet, histopathology training in epileptic disorders is difficult to obtain in many European countries. EpiCare offers, therefore, a comprehensive neuropathology service for all of their members. Currently, our workpackage addresses three activities:
1) The European Epilepsy Brain Bank is an EU-FP6/FP7 funded project collecting more than 10.000 histopathology diagnosis obtained from epilepsy surgery. This unprecedented dataset can be used for benchmark projects on disease etiologies, outcome and pathomechanisms.
2) We also offer a second diagnostic look to surgical brain samples obtained from epilepsy surgery using standardized protocols. Formalin-fixed and paraffin-embedded tissue specimens should be send to the neuropathology laboratory in Erlangen, Germany (see address below).
3) Finally, we offer an interactive, online post-surgical case-conference to discuss surgical brain specimens from various European epilepsy surgery centers. For further information, see sections below.
EU-FP6/FP7 funded European Epilepsy Brain Bank
The European Epilepsy Brain Bank (EEBB) has enrolled more than 10.000 epilepsy surgery patients from 36 centers in 14 European countries (Blümcke et al. 2017; Lamberink et al. 2020). EEBB is a virtual database collecting a minimal data set for each (double-encoded) patient, e.g. age at epilepsy surgery, age at seizure onset, sex, location of the epileptic lesion, year of surgery, 12-24-60 month postsurgical outcome, antiseizure medication). This unprecedented databank is used for benchmark projects on disease etiologies in focal epilepsy (Blümcke et al. 2017) and post-surgical outcome (Lamberink et al. 2020). Tissue storage will help to enable future research projects, in particular addressing genetic causes of focal brain lesions and epileptogenicity.
Neuropathology service for microscopic brain tissue review
Members of this workpackage offer the large experience in standardized neuropathological examination, i.e. establishing ILAE guidelines and classification systems. All tissue specimens will remain the courtesy of submitting centers and returned upon completion of the analysis. Guidelines for standardized tissue fixation, long-term preservation and storage have previously been published and can be downloaded through this website (Blümcke et al. 2016). Formalin-fixed and paraffin-embedded tissue specimens should be send for microscopic review to the postal address below.
Members
Homa Adle-Biassette, Paris
Eleonora Aronica, Amsterdam
Annmaria Buccoliero, Florence
Albert Becker, Bonn
Rita Garbelli, Milano
Kristof Egervari, Geneva
Maria Thom, London
Toumas Rauramaa, Kupio
Thomas Olsson Bontell, Gothenburg
Thomas Jacques, London
Leaders
Post-surgical case discussion
We offer a monthly online discussion forum for post-surgical patients in order to review the histopathology findings. The post-surgical discussion forum is targeted for clinicians, neurosurgeons and histopathologists. Each case will be presented with the patient’s clinical history and surgical procedure. The microscopy findings will be reviewed live using our digital microscopy platform. The discussion of differential diagnosis and postsurgical outcome will conclude each case presentation. If you are interested to join this forum, or present a case please email to bluemcke@uk-erlangen.de.
For patient presentation, we request to obtain the patient consent using the standardized EU form.
Summary
Approved and evidence based treatments do not exist for many of the rare and complex epilepsies. In addition to improve high quality evidence of clinical studies, there is an urgent need to identify and develop novel treatments targeting specific aetiologies. Recognition of the epilepsies as a group of rare diseases with known aetiologies give us insight as to the underlying mechanisms involved in both seizure generation, as well as comorbidities such as cognitive, physical, emotional and behavioural capacities. To improve study designs and choice of relevant interventions, there is a need to collect standardized information on outcomes associated with specific treatments such as anti-seizure medication (ASM) , repurposed drugs targeting underlying mechanisms, disease-modifying treatments (including immunosuppression, treatments targeting protein and gene expression), and non- pharmacological treatments (such as diets, neurostimulation, and surgical therapies) or interventions (cognitive, behavioral and physical therapies) in epilepsy patients. To guarantee advancements, interest groups for specific epilepsies (e.g. auto-immune epilepsies, tuberous sclerosis, etc), or with focus in specific aspects of trial design (e.g. n-of 1- methodology, use of PCOMs etc.) are required which identify the particular needs of an entity to enable conduction and ultimately performance of clinical trials.
Ongoing Special Interest Group
If you wish to contribute to the work of a SIG or propose the creation of a new one please contact Nicola Openshaw-Lawrence.

Leaders
Objectives
- To promote and facilitate the development of better treatments or interventions, possibly in the context of clinical trials for people with epilepsy
- To support and make newly undertaken academic treatment and intervention trials, designed in EpiCare affiliated centers known (among other PINPOINT project).
- To provide advice and professional expertise to any party involved in the conduction of academic clinical trials in epilepsy and related disorders, regarding trial design, statistical analysis, outcome measures including patient centered outcome measures (PCOM), regulatory and ethical aspects, in order to improve the quality and implementation of the results.
- To develop educational activities to empower parties with the knowledge necessary to develop and design clinical trials and studies with targeted treatments.
- To establish interest groups for specific epilepsies.
- To facilitate implementation of high quality evidence from clinical trials in daily clinical care by creation of clinical recommendations for targeted treatments.
The E-pilepsy pilot network of cooperation in epilepsy surgery
It has been operating since January 2014. It has provided a large number of deliverables and sustained activities that have been consolidated and further developed in EpiCARE. The network initially included 28 epilepsy surgery centres but has recently grown to 52 centres. While only some epilepsy centers offer comprehensive epilepsy care and can be labelled full members of EpiCARE, it is essential that the E-pilepsy pilot network of cooperation continues to expand and ensures optimal dissemination of information, guidelines, state-of-the-art methodologies and web-based tools which help increase the effectiveness and safety of pre-surgical evaluation and epilepsy surgery.
The Epilepsy Surgery workpackage comprises the following activities:
– 1) Monthly E-Care visioconferences: These conferences, which allowed to discuss complex epilepsy surgical cases, have successfully continued under the coordination of Stefano Francione. Starting April 2021 this activity expanded to bi-monthly meetings (every 2nd and 4th Wednesday of the month from 4pm to 5H30pm CET) to increase the number of discussed surgical cases. The regularly updated agenda of EpiCARE Case discussions can be found on: https://epi-care.eu/case-discussions-epicare/
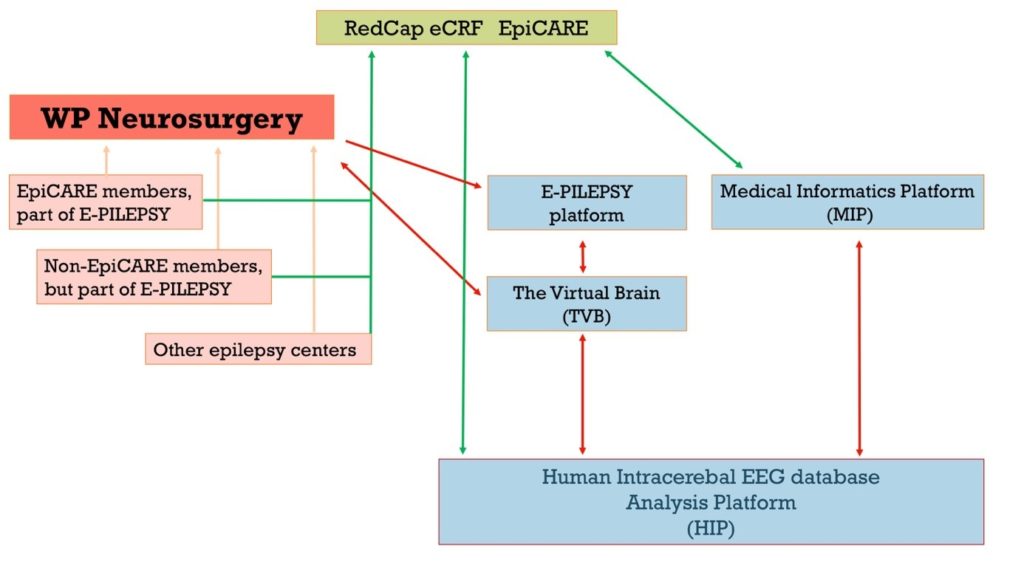
– 2) IT platform: The E-PILEPSY IT platform allowed each EpiCARE center to get access and use sophisticated neuroimaging and EEG postprocessing tools (e.g. Curry8). Maintenance includes supporting the data safety and data privacy requirements of the platform, including regular changes of each center password to access the platform via VPN (every 6 months). It will soon evolve into an expanded platform with additional functionalities, the Human Intracerebral EEG platform (HIP), thanks to the partnership established between EpiCARE and the Human Brain Project.
– 3) Epilepsy Surgery RedCap eCRF: This comprehensive eCRF aimed at collecting all relevant data from patients undergoing presurgical evaluation and epilepsy surgery. It is currently proposed to focus on patients undergoing invasive EEG recordings, but shall later expand to all patients considered for epilepsy surgery. Data can be entered on a local or central RedCap database. For those stored in their hospital of origin (local RedCap server), the Medical Informatics Platform (MIP), also developed within the framework of the Human Brain Project, can be used to federate the datasets without moving them away from their site of origin and storage.
– 4) The CogniEEG project: EpiCARE centers are core members of the CogniEEG project which aims at leveraging cognitive research performed in patients with epilepsy undergoing intracerebral EEG recordings (iEEG). Other iEEG centers from Europe, Asia and Oceania are also contributing to this project, which take advantage of the HIP platform and epilepsy surgery RedCap eCRF to collect, organise and analyse data.
– 5) The Registry: All data collected through the above activities (RedCap eCRF, CogniEEG and iEEG data in general) will feed the Epilepsy Surgery registry built to this purpose. The latter will communicate and cross-talk with the general EpiCARE registry, and will use the unique EUPID identifier for all EU patients.
Leaders
Objectives
- Consolidate and continue the E-pilepsy pilot network of cooperation in epilepsy surgery
- Expand the number and participants to the E-Care visioconferences on epilepsy surgery,
- Further develop the IT platform devoted to pre-surgical post-processing and visioconferences, and make available for use by other workpackages in EpiCARE
- Promote the dissemination and use of the E-Database devoted to epilepsy surgery
- Upgrade information on epilepsy surgery and E-eligibility tools on the web-site
- Expand and upgrade e month 12pilepsy surgery guidelines
Healthcare & research policies
Summary
Neonatal seizures are a major diagnostic challenge for clinicians because clinical presentations can be very subtle, electro-clinical correlation variable and there is generally a poor response to antiepileptic drugs. Interpretation of neonatal EEG is a highly specialised task and a thorough knowledge of the maturational changes that exist across all gestational ages is required. In addition, this expertise must be available on a 24/7 basis to meet the needs of the neonatal intensive care unit environment. Over the last number of years, we have developed the Babylink platform (G Boylan), a web based IT platform for streaming neonatal EEG on a 24 hour basis where it can be interrogated by novel neonatal seizure detection algorithms. This platform provides support to hospitals where EEG expertise is not readily available. Through the EpiCARE network we aim to scale this platform to a European population of neonates with seizures and we will identify a group of experts from the ERN centres who will be available to provide oversight for the platform
Leaders

Dr. Ronit Pressler
London, UK

Pr. Carmen Fons
Barcelona, Spain
Objectives
- To establish a European neonatal seizures expert group
- To provide guidelines for EEG monitoring in neonates with acute seizures and epilepsies across Europe
- To scale up the neonatal EEG remote monitoring platform (Babylink) for use across Europe
- To develop management guidelines and treatment protocols for neonatal seizures
- To define and implement standardised outcomes of neonatal seizures that can be applied across EpiCARE
- To provide a web base educational toolkit for neonatal seizures and epilepsies specifically for health professionals, families and public interest groups
- To establish a neonatal seizure registry in Europe and promote collaborative research
Summary
Strengthening the links between EpiCARE and existing, or under development, national epilepsy networks and pathways for optimal epilepsy care is one of our priorities.
The governance structure of ERN EpiCARE that facilitates, pooling and sharing knowledge, fostering research and innovation, as well as the discussion on rare or low prevalence complex epilepsies is now in place. To reap all benefits for patients, effective mechanisms to connect EpiCARE medical teams with national health systems need to be developed. The National Networks WG aim is to reinforce building national/regional epilepsy care pathways to ensure timely referral to the right level of care, systematic follow-up, awareness of treatment options and self- care, good communication between the different actors and flexible consultation possibilities including national case discussions before referring the case to the ERN-level discussions. National networks need also to include peer support to reinforce care and a service plan, where appropriate. Our upcoming tasks are:
- to complete the mapping of the current situation of national epilepsy care pathways in EU countries and to provide a thorough analysis of the mapped pathways to identify strengths, weaknesses, gaps, and opportunities for building better epilepsy care pathways;
- to harmonize minimum requirements for level 3 and 4 epilepsy centres within the EU; via systematic literature review and Delphi process among EpiCARE ERN members;
- to reinforce national and regional activities to link EpiCARE ERN to the national health care systems serving epilepsy patients.
Our actions also take into consideration the ILAE-IBE recommendations for an integrated approach to «epilepsy and other neurological disorders» (WHO iGAP) and contribute to actions taken at the level of the EU member states, within the frame of the Direct-Action grant, JARDIN, funded by the Commission.
Leaders
Registry project
Summary
One of the aims of the EpiCARE network will be to identify the currently existing registries and databases on rare and complex epilepsies in Europe. Many institutions, networks as well as patient organizations have already developed or are in the process of developing a database with key information on patients with a specific epilepsies, at both national and European levels. Notable examples of organizations with rather well developed databases are the Dravet Syndrome European Federation, and Tuberous Sclerosis. Some organizations also gather crucial patient information through questionnaires. After identification of databases, a next step will be to look for common denominators and to merge some of the common topics across the datasets. This will allow us to better estimate and describe the true incidence of typical disease characteristics. It will for instance be possible to find common co-morbidities (such as depression, memory problems, and cognitive problems) across many rare and complex epilepsies. Efficacy and side effects of older and newer treatment options can be compared for different epilepsies. We will also determine whether the existing or newly developed guidelines by EpiCARE are also applicable for specific epilepsy syndromes. At the research level, managing the databases and registries will allow us to recognize specific patient groups for advanced diagnostic and therapeutic studies and/or for answering specific research questions.
One of the final deliverables of this work package will be to construct a common “minimum E-database” with key disease features. This electronic database will be disseminated for use in the network which finally will lead to better and more standardized patient care. The platform we will use to build this E-database is REDCap (Research Electronic Data Capture), already utilised by E-pilepsy.
Leaders
Objectives
- Identification of exiting databases / registries for rare and complex epilepsies
- Linking of databases / registries
- Building E-database with key clinical features
Guidelines
Summary
The development of clinical guidelines and care pathways is crucial to improve the access to diagnosis and treatment. Guideline development was a key deliverable of E-pilepsy; such will be essential to the provision of high-quality healthcare to all patients with rare and complex epilepsies. Guidelines should also be focal points for medical training and research. We will continue to use a transparent methodology (Grading of Recommendations Assessment, Development and Evaluation; GRADE) in order to ensure implementation of all available evidence, and involvement of all stakeholders. All developments will follow the rules of the GRADE Methodology, which uses the best available evidence along with expert opinion and patient representatives to develop explicit criteria to guide clinical care. Systematic reviews of relevant evidence will thus precede all further activities in this WP and will underpin all medical information, procedures, and applications developed in EpiCARE.
“Epilepsies in children and young people: Investigative procedures and management” is the new guideline reflecting the most recent evidence around key issues and replaces SIGN 81.
- To assess adherence to existing guidelines in Centres of the European Pilot reference Network E-pilepsy and the EpiCARE network
- To determine gaps in areas of need of development of guidelines in epilepsy care of rare and complex drug resistant epilepsies, by undertaking systematic reviews of the relevant topics at stake
- To develop clinical care guidelines together with the relevant medical societies and representatives of health care providers and patient groups
Leaders

Pr. Eugen Trinka
Salzburg, Austria

Pr. Federico Vigevano
Rome, Italy

Dr. Nicola Specchio
Rome, Italy

Dr. Teia Kobulashvili
Salzburg, Austria
Objectives
- To identify international epilepsy clinical care guidelines and assess their quality by using a systematic methodology.
The EpiCARE Guideline Development Group conducted a survey with the aim to evaluate the current state of the guidelines being used by centres of the EpiCARE network for diagnosis and treatment of rare and complex epilepsies. The guideline group is currently assessing the quality of collected documents by using the AGREE II tool. The results of the survey will be published in due course.
A considerable number of educational activities and tools are already available in most EU countries, the result of either individual academic initiatives or as part of programs developed by the scientific societies (ILAE, EPNS, EAN) developed by experts from the participating centres.. What is still challenging, due to the great variety of “end-users” in the field, is the development of learning goals, preferably per “end-user”, and the establishment of priorities.
EpiCARE is an EU reference network of all disciplines involved in epilepsy care for rare and complex epilepsies. For general and specialized training in epileptology we fully adhere to the “Roadmap for a competency-based educational curriculum”, developed by the International League Against Epilepsy. In parallel, as part of a reference network of Health Care Providers for rare and complex epilepsies, the first goal of the present Working Group is to develop educational practices favouring dissemination of highly specialized knowledge and expertise within the members of the ERN and beyond, to operationalise complementarity.
With the generous contribution of experts in the corresponding fields, we developed a program of educational webinars. They offer comprehensive state-of-the-art reviews, accessible to all free of charge. At first presentation, all our webinars also offer a “meet the expert” opportunity during a live Q&A part.
Supported by a European Commission CEF Telecom program (2018 and 2021) we are also developing, in close collaboration with the ILAE Academy, a series of patient-centred interactive e-learning modules. Each of them offers a comprehensive diagnostic and therapeutic, state-of-the-art knowledge and interactive training.
Our Clinical Case Discussion weekly sessions, strictly reserved for confidentiality reasons to pre-registered health care professionals, are another source of education.
EpiCARE members regularly contribute to “Seminars in Epileptology” review manuscripts, published by the ILAE educational journal Epileptic Disorders.
Finally, HCPs members of the ERN EpiCARE in close collaboration with our Patient Advocacy Groups, regularly develop and disseminate leaflets for caregivers and patients with rare epilepsies and comprehensive patient’s journeys descriptions.
Training in clinical epileptology and related disciplines requires the physical presence of young clinicians and researchers at epilepsy reference centres. All ERN EpiCARE members and collaborating partners offer training possibilities. They can be directly contacted by those interested.
We are also partnering the Mobility Program developed and funded by the European Commission. Considering the major role of epilepsy nurses and epilepsy monitoring unit nurses we support short exchange programs between HCPs members of EpiCARE. AN EpiCARE Task Force is currently focusing upon the development of training guidelines for paramedics in epilepsy departments.
The ERN EpiCARE is one of the 3 pilot ERNs, missioned to contribute to the future development of an ERN ACADEMY and an active member of the ERNs Knowledge Generation Working Group, focusing upon the development of a joint strategy of the ERNs for training and education to overcome the gap between the increasing awareness on rare and complex diseases and the lack of a specific training in the medical curricula.
Leaders
Active members:
Arzimanoglou Alexis, France
Ingmar Blümcke, Germany (ILAE Academy Liaison)
Jansen Floor, The Netherlands
Kalviainen Reetta, Finland
Kobulashvili Teia, Austria (Liaison Guidelines WP)
Lagae Lieven, Belgium
Lesca Gaetan, France
Liakina Tatjana, Lithuania
Malenika Masa, Croatia
Openshaw-Lawrence Nicola, UK (Epilepsy Nurse liaison)
Papadopoulou Marietta, Greece
Pressler Ronit, UK (Liaison, Neonatal WP)
Specchio Nicola, Italy
Toulouse Joseph, France
Vigevano Federico, Italy
Zuberi Sameer, UK
Research is an important component of EpiCARE. Genetic research, genetic discovery, and improved diagnosis of rare and complex genetic syndromes with epilepsy are at the forefront of the activities of this workpackage.
Various EpiCARE partners coordinating and participating in the activities of this workpackage. They conveyed into it the knowledge and opportunities that have derived by their participation as active members to major concluded and existing EU research networks on epilepsy research in genetics and rare epileptic encephalopathies, which include:
- the FP7 project ‘DESIRE’ (Development and Epilepsy – Strategies for Innovative Research to improve diagnosis, prevention and treatment in children with difficult to treat Epilepsy- grant agreement no. 602531), has provided important contributions to the study of complex and rare epileptic encephalopathies and has collected DNA and brain tissue samples (Blumcke I, Spreafico R, Haaker G, et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N Engl J Med. 2017;377(17):1648-1656. doi:10.1056/NEJMoa1703784), cohorts with rare syndromes, organized databases and clinical trials;
- the Epicluster initiative of the European Brain Research Area (EBRA), which is subserving a collaborative framework for the coordinated actions of epilepsy research in Europe, based around shared partnerships and research priorities;
- the Neuro-MIG European Network on Brain Malformations (CA16118 Funded by the European Cooperation in Science and Technology Action – COST), which has created a powerful international network dedicated to the Genetic diagnosis and management of brain malformations;
- the Rare Epilepsy Syndromes (RES) consortium.
To boost the genetic research activities, the ERN EpiCARE contributed to the development of a web-platform for patients’ matchmaking, interoperable with other discovery services, based on the RD-NEXUS tool, and provided by the European Joint Project in Rare Disease (EJPRD) to support the wider European Reference Network (ERN) community. This approach, which has been developed by Dr. Davide Mei at Meyer Hospital in Florence with the support of Prof. Anthony Brooks of the University of Leicester, will make the patient’s metadata and core data queryable by the users, thus allowing the discovery of relevant clusters homogeneous for clinical and/or genetic features.
Finally, ERN-EpiCARE has recently joined, as an associated ERN to Solve-RD, a Horizon2020-funded project, dedicated to “solve the unsolved rare diseases”. This will permit the re-analysis of unsolved exomes and genomes of patients with rare developmental and epileptic encephalopathy (DEE) or other rare epilepsy syndromes.
The WP15 is a transversal working package closely collaborating with all other EpiCARE WPs. Particularly WP2 on Diagnostics, WP6 on Neuropathology, WP7 on Targeted therapies and Clinical Trials, WP8 on Epilepsy Surgery.
Together with the Education and Training WP14, following an initiative of Pr Lesca, more than 20 educational webinars focusing on genetic epilepsies were already released starting June 2020. The presentations systematically combine a clinician and a geneticist. WP15 is represented by Pr. Guerrini at the EpiCARE Research Council.
The WP15 also contributed to the creation of a collaborative genetic research plateform, with the aim of recruiting European series of patients carrying variants in already known genes but with limited knowledge that need to be expanded.
Leaders

Pr. Renzo Guerrini
Florence, Italy

Dr. Simona Balestrini
Florence, Italy
Workpackage 11 – Case discussions
Summary
Case discussions are an integral part of all ERNs and are in line with the European Commission’s Directive. EpiCARE undertakes regular case discussions in specialised areas of the rare and complex epilepsy field, including surgical and non-surgical cases, neuropsychology and neuropathology.
These discussions utilise the Clinical Patient Management System (CPMS) which is an online secure and encrypted platform that allows for free-flowing discussion amongst experts, enabling complex patient cases to have multi expert input and therefore optimise the care and treatment of rare and complex epilepsy patients.
There are two ways in which cases can be discussed and these are in an online meeting area within the CPMS platform or via written contributions, both avenues allow for detailed examination of each case and feedback provision.
The members of WG11 are working to increase attendance and participation in these case discussions and to highlight areas within the rare and complex epilepsy field that would benefit from these multi-disciplinary discussions.
The primary goal of ERN EpiCARE is to help the patient community which we serve, and these case discussions support this goal and also guide further practice and treatment in our expert centres.
Leaders
Objectives
Psychosocial issues, quality of life, patient perspectives (PROMS), sexuality, ASM adherence and many others, are essential to a comprehensive care of the epilepsies, independently of age and seizure severity.
The main aim of WG18 is to work on issues related to global epilepsy care. Such as:
- Comorbidities in general;
- Psychiatric, behavioural and emotional regulation issues;
- Sexual dysfunction;
- ASM adherence-issues;
- Lifestyle and seizure-precipitating factors;
- HRQOL-issues;
- Patient reported outcomes (PROMS).
- Social integration issues.
Following a survey on “Use of screening tools to assess comorbidities and adverse events in patients with epilepsy. A European Reference Network for Rare and Complex Epilepsies (EpiCARE) survey” (Henning et al. 2022), WG18 was initiated by a group of adult neurologists from Norway (M. Lossius – Chair), K. Åshild Alfstad, O.J. Henning), Denmark (G. Rubboli), Finland (R. Kälviäinen, J. Peltola) and Germany (Ch. Helmstaedter, H. Hamer, A. Schulze-Bonhage, B. Steinhoff). After the face-to-face 4th “In search of lost time” workshop, held in Rome (December 2023), the WG18 expanded to include child neurologists. They are expected to contribute to all challenges mentioned above and focus on issues related to intellectual disabilities, leaded by S. Jozwiak (Poland).
Very recently a Special Interest Group (SIG) on Transition was created, lead by V. De Giorgis (Italy) and M. Malenica (Croatia). A survey was launched, aiming to have a “photo” of the practices in EpiCARE centres and on how our members deal with transition from childhood to adolescence and adulthood of patients with: – Epileptic & Developmental Encephalopathies; – Drug-resistant Juvenile Myoclonic Epilepsy or focal idiopathic/LKS/CSWSS; – Drug-resistant Focal Structural Epilepsies that could not benefit from surgery (following pre-surgical evaluation) or already operated on without a full control of seizures or persisting comorbidities.
Leaders
Summary
Partnership between medical experts in rare and complex epilepsies with families and patients suffering from them is an important mission of EpiCARE.
European Patients Advocates (ePAGs) for epilepsy care are an independent body of patient representatives, who are either leaders in rare and complex epilepsy patient organisations from across Europe, or patients themselves. The group currently consists of 15 members (https://epi-care.eu/epicare-patient-representatives/), from various European countries representing different conditions. They are the voice of patients for EpiCARE, produce deliverables to support patients and their families, facilitate communication between patients and healthcare professionals. The patient empowerment working groups main mission is to better coordinate interactions between the patient advocates group and the EpiCARE medical teams by:
- Reinforcing the links between existing and under development federations and associations of patients with rare and/or complex epilepsies, the EU chapters of the International Bureau for Epilepsy (IBE) and the ERN EpiCARE community.
- Supporting the patient associations to better defend the need for national and EU plans for epilepsy care. This is of particular importance in the coming years – in a joint effort with EpiCARE and the European chapters of the ILAE and the IBE – aiming to adapt and implement in Europe the Intersectoral Global Action plan (iGAP) on «epilepsy and other neurological disorders (2022-2031) adopted by the WHO Assembly.
- Incorporating the voice of patients from the different EpiCARE WGs and collaborating to develop information, dissemination, guidelines, and support to research projects.
- Pursue, in collaboration with EpiCARE experts, with projects that increase disease knowledge for patients, families and non-expert treating physicians such as: Webinars, definition of Patients Journeys, Leaflets, and publications in scientific journals.
Leaders

Isabella Brambilla
Patient representative
ePAG chair
Dravet Onlus, Italy
Epilepsy Nurses
Summary
Partnership between medical experts in rare and complex epilepsies with families and patients suffering from them is an important mission of EpiCARE.
This WG was made a part of the ERN EpiCARE programme in late 2022. Its creation results from the very successful exchange program (2020-22), funded by the management budget of EpiCARE. It was launched during a workshop held in Utrecht (06/23) at the EpiCaRE annual meeting, involving nurses and VEEG technicians working in the field of epilepsy care and epilepsy-monitoring units. The upcoming tasks are:
-Identify the difference in monitoring practices in different HCPs
- Undertake a literature review of monitoring practices and the differences in units/centres in the EU
- Compare and contrast monitoring practice guidelines from ERN EpiCare centres
- Prepare a journal paper (for submission to an appropriate journal) with findings and recommendations.
– Produce a journal paper on the reasonings behind the launch of this WG and its benefits and aims. The WG works in close collaboration with the Nursing section of the ILAE (https:// www.ilae.org/nursing-section).
– Assess the learning and practice needs of epilepsy nurses and EEG technicians working in Europe;
– Contribute to the development, and adapt to European standards and needs, of an education curriculum.
– Learn from each other and improve patient care.
Leaders

Nicola Lawrence
EpiCARE management team
Data Manager & CPMS coordinator
London, UK
For more information, contact: helpdesk@epi-care.eu.